

Shandong Science ›› 2023, Vol. 36 ›› Issue (5): 102-120.doi: 10.3976/j.issn.1002-4026.2023.05.013
• Environment and Ecology • Previous Articles Next Articles
HE Zhenbo( ), ZHANG Li, GAO Mingxin, LUAN Lingyu(
), ZHANG Li, GAO Mingxin, LUAN Lingyu( )
)
Received:2022-10-29
Published:2023-10-20
Online:2023-10-12
Contact:
LUAN Lingyu
E-mail:10431201072@stu.qlu.edu.cn;sdlly916@126.com
CLC Number:
HE Zhenbo, ZHANG Li, GAO Mingxin, LUAN Lingyu. Research progress of green scale inhibitors for circulating cooling water[J].Shandong Science, 2023, 36(5): 102-120.
Table 1
Structures and characteristics of traditional chemical scale inhibitors"
| 阻垢剂 | 化学结构式 | 主要特性 |
|---|---|---|
| 2-膦酸基-1,2,4-三羧酸丁烷 (PBTCA)[ | 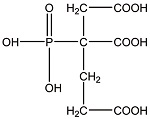 | 有机膦酸,含有羧酸和膦酸官能团,与Ca2+有较好的络合能力,具有良好的阻垢、分散性能和耐高温性能,可作螯合剂和分散剂。 |
| 羟基乙叉二膦酸 (HEDP)[ | 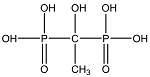 | 有机膦酸,含有膦酸和羟基官能团,能与Ca2+生成稳定的络合物,具有良好的阻垢缓蚀性能,毒性小和抗氧化性强,可作阻垢缓蚀剂和螯合剂。 |
| 氨基三亚甲基膦酸 (ATMP)[ | 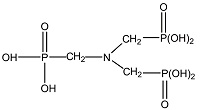 | 有机膦酸,含有膦酸官能团,化学性质稳定且较难水解,对Ca2+具有良好的螯合能力,在较低浓度时就引起晶格畸变,具有良好的阻垢性能和阈值效应。 |
| 乙二胺四乙酸 (EDTA)[ | 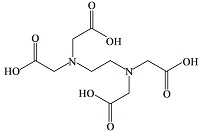 | 含有羧酸官能团,能与Ca2+形成稳定的络合物,是螯合剂的典型代表物质,常被用作螯合剂和滴定指示剂。 |
| 聚丙烯酸 (PAA)[ | 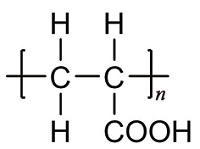 | 水溶性聚羧酸类阻垢剂,含有羧基,能与Ca2+螯合,具有阻垢和分散性能,不可降解。 |
| 氨三乙酸 (NTA)[ | 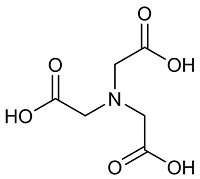 | 含有羧酸官能团,单个分子能提供4个配位键,具有非常强的络合能力,可以与Ca2+形成稳定的螯合物,可作阻垢剂和洗涤剂。 |
Table 2
Structures and characteristics of main elements of natural organic scale inhibitors"
| 阻垢剂 | 化学结构式 | 主要特性 | |||
|---|---|---|---|---|---|
| 生姜提取物[ | 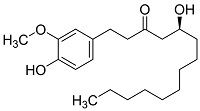 | 含有羟基和羰基等官能团,羟基可与Ca2+螯合,对钙垢的形成有一定的抑制作用 | |||
| 褐藻提取物[ | 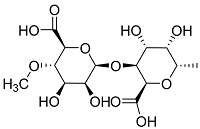 | 褐藻酸含有羧基和羟基等官能团,对Ca2+有很强的结合能力,具有良好的阻垢性能,可作阻垢剂和食品添加剂 | |||
| 橄榄叶提取物[ | 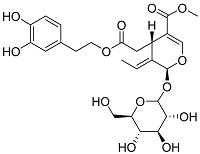 | 橄榄苦柑含有羟基和酯基等官能团,能够络合Ca2+,具有良好的阻垢性能 | |||
| 萝卜提取物[ | 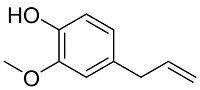 | 提取物中可与Ca2+相互作用的主要化合物的活性官能团有羟基和羧基,醚键会提高聚合物在钙垢晶体的吸附性 | |||
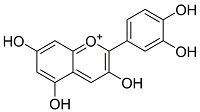 | |||||
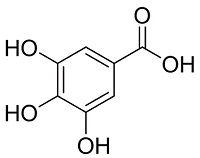 | |||||
| 单宁酸[ | 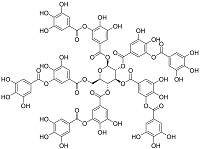 | 多酚类化合物,无磷且易降解,含有酚羟基、羰基等官能团,易与Ca2+形成可溶性较大的螯合物 | |||
| 柠檬酸[ | 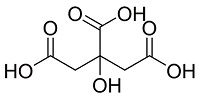 | 环境友好型天然有机酸,结构中含有羧基和羟基,能与Ca2+螯合,具有阻垢性能,可用作阻垢剂、芳香剂和防腐剂 | |||
| 淀粉[ | 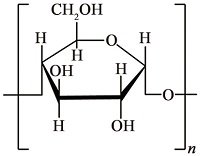 | 天然有机高分子阻垢剂,含有羟基和醚键官能团,能够络合Ca2+,具有一定的阻垢性能,但自身阻垢效果不够好,高温下易分解 | |||
| 壳聚糖[ |  | 天然多糖,无毒生物可降解,含有羟基和氨基官能团,能与Ca2+发生螯合反应,具有阻垢和缓蚀性能,但分散性能和抗菌活性差 | |||
Table 3
Structures and characteristics of green scale inhibitors"
| 阻垢剂 | 化学结构式 | 主要特性 |
|---|---|---|
| 聚天冬氨酸[ | 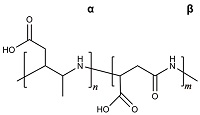 | 无磷无毒,具有生物可降解性,侧链含有羧基官能团,可以螯合Ca2+,主要通过改变钙垢晶体结构来抑制钙垢的生长,具有良好的阻垢性能,但在高温或高硬度水体中阻垢效果不够好。 |
| 聚环氧琥珀酸[ |  n=2~10 M为Na+或H+,K+,NH4+ R为H或C1-4烷基 | 无磷无毒,生物降解性好,能与Ca2+螯合,主要通过延缓晶体成核时间来抑制钙垢的形成,可做阻垢缓蚀剂。但存在投加量大、Ca2+耐受性差和耐温性差等缺点。 |
| 苹果酸[ | 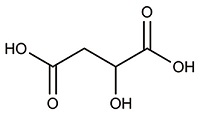 | 无毒,生物可降解,含有羧基和羟基官能团,具有一定的阻垢性能,可作食品添加剂和阻垢剂。 |
| 羧基甲菊粉[ | 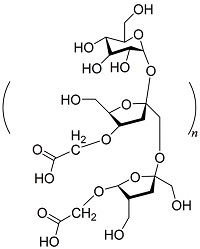 | 属于多糖,无毒,不含氮、磷,具有可再生和生物降解性,含有羧基和羟基官能团,可以与Ca2+发生螯合反应,引起晶格畸变,能够有效地抑制钙垢的形成。可用于石油勘探、药物输送和抑制钙垢。 |
| [1] | RAHMANI K, JADIDIAN R, HAGHTALAB S. Evaluation of inhibitors and biocides on thecorrosion, scaling and biofouling control of carbon steel and copper-nickel alloys in a power plant cooling water system[J]. Desalination, 2016, 393: 174-185. DOI: 10.1016/j.desal.2015.07.026. |
| [2] | ZHU T Z, WANG L D, SUN W, et al. The role of corrosion inhibition in the mitigation of CaCO3 scaling on steel surface[J]. Corrosion Science, 2018, 140: 182-195. DOI: 10.1016/j.corsci.2018.06.003. |
| [3] | ZHAO Y Z, JIA L L, LIU K Y, et al. Inhibition of calcium sulfate scale by poly (citric acid)[J]. Desalination, 2016, 392: 1-7. DOI: 10.1016/j.desal.2016.04.010. |
| [4] | CAN H K, NER G. Water-soluble anhydride containing alternating copolymers as scale inhibitors[J]. Desalination, 2015, 355: 225-232. DOI: 10.1016/j.desal.2014.11.001. |
| [5] | WANG C, SHEN T, LI S, et al. Investigation of influence of low phosphorous co-polymer antiscalant on calcium sulfate dihydrate crystal morphologies[J]. Desalination, 2014, 348: 89-93. DOI: 10.1016/j.desal.2014.06.017. |
| [6] | MIGAHED M A, RASHWAN S M, KAMEL M M, et al. Synthesis, characterization of polyaspartic acid-glycine adduct and evaluation of their performance as scale and corrosion inhibitor in desalination water plants[J]. Journal of Molecular Liquids, 2016, 224: 849-858. DOI: 10.1016/j.molliq.2016.10.091. |
| [7] | LIU G Q, ZHOU Y M, HUANG J Y, et al. Acrylic acid-allylpolyethoxy carboxylate copolymer as an effective inhibitor for calcium phosphate and iron(III) scales in cooling water systems[J]. CLEAN - Soil, Air, Water, 2015, 43(7): 989-994. DOI: 10.1002/clen.201100569. |
| [8] | BUTT F H, RAHMAN F, BADURUTHAMAL U. Evaluation of SHMP and advanced scale inhibitors for control of CaSO4, SrSO4, and CaCO3 scales in RO desalination[J]. Desalination, 1997, 109(3): 323-332. DOI: 10.1016/s0011-9164(97)00078-7. |
| [9] | ZUO Z, YANG W, ZHANG K, et al. Effect of scale inhibitors on the structure and morphology of CaCO3 crystal electrochemically deposited on TA1 alloy[J]. Journal of Colloid and Interface Science, 2020, 562: 558-566. DOI: 10.1016/j.jcis.2019.11.078. |
| [10] | JI Y, CHEN Y, LE J X, et al. Highly effective scale inhibition performance of amino trimethylenephosphonic acid on calcium carbonate[J]. Desalination, 2017, 422: 165-173. DOI: 10.1016/j.desal.2017.08.027. |
| [11] | LUPU C, ARVIDSON R S, LUTTGE A, et al. Phosphonate mediated surface reaction and reorganization: Implications for the mechanism controlling cement hydration inhibition[J]. Chemical Communications (Cambridge, England), 2005(18): 2354-2356. DOI: 10.1039/b500192g. |
| [12] |
LI X, GAO B, YUE Q, et al. Effect of six kinds of scale inhibitors on calcium carbonate precipitation in high salinity wastewater at high temperatures[J]. Journal of Environmental Sciences, 2015, 29: 124-130. DOI: 10.1016/j.jes.2014.09.027.
pmid: 25766020 |
| [13] | SHEIKHI A, LI N, VAN DE VEN T G M, et al. Macromolecule-based platforms for developing tailor-made formulations for scale inhibition[J]. Environmental Science: Water Research & Technology, 2016, 2(1): 71-84. DOI: 10.1039/C5EW00158G. |
| [14] | ZUO Y W, SUN Y, YANG W Z, et al. Performance and mechanism of 1-hydroxy ethylidene-1, 1-diphosphonic acid and 2-phosphonobutane-1, 2, 4-tricarboxylic acid in the inhibition of calcium carbonate scale[J]. Journal of Molecular Liquids, 2021, 334: 116093. DOI: 10.1016/j.molliq.2021.116093. |
| [15] | GUO X R, QIU F X, DONG K, et al. Preparation and application of copolymer modified with the palygorskite as inhibitor for calcium carbonate scale[J]. Applied Clay Science, 2014, 99: 187-193. DOI: 10.1016/j.clay.2014.06.031. |
| [16] | SHAKKTHIVEL P, VASUDEVAN T. Acrylic acid-diphenylamine sulphonic acid copolymer threshold inhibitor for sulphate and carbonate scales in cooling water systems[J]. Desalination, 2006, 197(1/2/3): 179-189. DOI: 10.1016/j.desal.2005.12.023. |
| [17] | AMJAD Z, KOUTSOUKOS P G. Evaluation of maleic acid based polymers as scale inhibitors and dispersants for industrial water applications[J]. Desalination, 2014, 335(1): 55-63. DOI: 10.1016/j.desal.2013.12.012. |
| [18] | YANG L, YANG W, XU B, et al. Synthesis and scale inhibition performance of a novel environmental friendly and hydrophilic terpolymer inhibitor[J]. Desalination, 2017, 416: 166-174. DOI: 10.1016/j.desal.2017.05.010. |
| [19] | CHAUSSEMIER M, POURMOHTASHAM E, GELUS D, et al. State of art of natural inhibitors of calcium carbonate scaling: a review article[J]. Desalination, 2015, 356: 47-55. DOI: 10.1016/j.desal.2014.10.014. |
| [20] | ZHANG H X, WANG F, JIN X H, et al. A botanical polysaccharide extracted from abandoned corn stalks: modification and evaluation of its scale inhibition and dispersion performance[J]. Desalination, 2013, 326: 55-61. DOI: 10.1016/j.desal.2013.07.015. |
| [21] | LIU D, DONG W B, LI F T, et al. Comparative performance of polyepoxysuccinic acid and polyaspartic acid on scaling inhibition by static and rapid controlled precipitation methods[J]. Desalination, 2012, 304: 1-10. DOI: 10.1016/j.desal.2012.07.032. |
| [22] | SHI W Y, DING C, YAN J L, et al. Molecular dynamics simulation for interaction of PESA and acrylic copolymers with calcite crystal surfaces[J]. Desalination, 2012, 291: 8-14. DOI: 10.1016/j.desal.2012.01.019. |
| [23] | GUO X, QIU F, DONG K, et al. Preparation, characterization and scale performance of scale inhibitor copolymer modification with chitosan[J]. Journal of Industrial and Engineering Chemistry, 2012, 18(6): 2177-2183. DOI: 10.1016/j.jiec.2012.06.015. |
| [24] | ZHANG B, ZHOU D, LV X, et al. Synthesis of polyaspartic acid/3-amino-1H-1, 2, 4-triazole-5-carboxylic acid hydrate graft copolymer and evaluation of its corrosion inhibition and scale inhibition performance[J]. Desalination, 2013, 327: 32-38. DOI: 10.1016/j.desal.2013.08.005. |
| [25] | LOURTEAU T, BERRICHE H, KÉCILI K, et al. Scale inhibition effect of Hylocereus undatus solution on calcium carbonate formation[J]. Journal of Crystal Growth, 2019, 524: 125161. DOI: 10.1016/j.jcrysgro.2019.125161. |
| [26] | LI S L, QU Q, LI L, et al. Bacillus cereus s-EPS as a dual bio-functional corrosion and scale inhibitor in artificial seawater[J]. Water Research, 2019, 166: 115094. DOI: 10.1016/j.watres.2019.115094. |
| [27] | MADY M F, KELLAND M A. Study on various readily available proteins as new green scale inhibitors for oil field scale control[J]. Energy & Fuels, 2017, 31(6): 5940-5947. DOI: 10.1021/acs.energyfuels.7b00508. |
| [28] | CASTILLO L A, TORIN E V, GARCIA J A, et al. New product for inhibition of calcium carbonate scale in natural gas and oil facilities based on Aloe vera: Application in venezuelan oilfields[C]// All Days. Cartagena de Indias, Colombia: SPE, 2009. DOI: 10.2118/123007-ms. |
| [29] | ABDEL-GABER A M, ABD-EL-NABEY B A, KHAMIS E, et al. A natural extract as scale and corrosion inhibitor for steel surface in brine solution[J]. Desalination, 2011, 278(1/2/3): 337-342. DOI: 10.1016/j.desal.2011.05.048. |
| [30] | ABD-EL-KHALEK D E, ABD-EL-NABEY B A, ABDEL-KAWI M A, et al. Investigation of a novel environmentally friendly inhibitor for calcium carbonate scaling in cooling water[J]. Desalination and Water Treatment, 2016, 57(7): 2870-2876. DOI: 10.1080/19443994.2014.987174. |
| [31] | KHALED R H, ABDEL-GABER A M, RAHAL H T, et al. A potential green anti-scaling and corrosion inhibitor for mild steel in brine solution[J]. International Journal of Electrochemical Science, 2020, 15(7): 6790-6801. DOI: 10.20964/2020.07.54. |
| [32] | AIDOUD R, KAHOUL A, NAAMOUNE F. Inhibition of calcium carbonate deposition on stainless steel using olive leaf extract as a green inhibitor[J]. Environmental Technology, 2017, 38(1): 14-22. DOI: 10.1080/09593330.2016.1183716. |
| [33] | GHIZELLAOUI S, BOUMAGOURA M, RHOUATI S, et al. Inhibition of CaCO3 growth in hard water by quercetin as green inhibitor[J]. Water and Environment Journal, 2020, 34(S1): 263-272. DOI: 10.1111/wej.12524. |
| [34] | MOHAMMADI Z, RAHSEPAR M. The use of green Bistorta Officinalis extract for effective inhibition of corrosion and scale formation problems in cooling water system[J]. Journal of Alloys and Compounds, 2019, 770: 669-678. DOI: 10.1016/j.jallcom.2018.08.198. |
| [35] | KIRBOĞ A S, ÖNER M. Inhibition of calcium oxalate crystallization by graft copolymers[J]. Crystal Growth & Design, 2009, 9(5): 2159-2167. DOI: 10.1021/cg800802z. |
| [36] | HAMDONA S K, EL-AASSAR A H M, AHMED A E M M, et al. Enhancing anti-scaling resistances of aromatic polyamide reverse osmosis membranes using a new natural materials inhibitor[J]. Chemical Engineering and Processing-Process Intensification, 2021, 164: 108404. DOI:10.1016/j.cep.2021.108404. |
| [37] | KHAMIS E, ABD-EL-KHALEK D E, ABDEL-KAWI M A, et al. New application of brown sea algae as an alternative to phosphorous-containing antiscalant[J]. Environmental Technology, 2022, 43(4): 595-604. DOI: 10.1080/09593330.2020.1797898. |
| [38] | VASYLIEV G, VOROBYOVA V, ZHUK T. Raphanus sativus L. extract as a scale and corrosion inhibitor for mild steel in tap water[J]. Journal of Chemistry, 2020, 2020: 1-9. DOI: 10.1155/2020/5089758. |
| [39] | CUI C C, ZHANG S G. Preparation, characterization and performance evaluation of a novel scale inhibiting and dispersing copolymer containing natural tannin[J]. Journal of Polymers and the Environment, 2020, 28(7): 1869-1879. DOI: 10.1007/s10924-020-01730-x. |
| [40] | CHEN Y, CHEN X S, LIANG Y N, et al. Synthesis of polyaspartic acid-oxidized starch copolymer and evaluation of its inhibition performance and dispersion capacity[J]. Journal of Dispersion Science and Technology, 2021, 42(13): 1926-1935. DOI: 10.1080/01932691.2020.1791172. |
| [41] |
XU Z, ZHAO Y, WANG J, et al. Inhibition of calcium carbonate fouling on heat transfer surface using sodium carboxymethyl cellulose[J]. Applied Thermal Engineering, 2019, 148: 1074-1080.
doi: 10.1016/j.applthermaleng.2018.11.088 |
| [42] | SHAHINI M H, RAMEZANZADEH B, MOHAMMADLOO H E. Recent advances in biopolymers/carbohydrate polymers as effective corrosion inhibitive macro-molecules: a review study from experimental and theoretical views[J]. Journal of Molecular Liquids, 2021, 325: 115110. DOI: 10.1016/j.molliq.2020.115110. |
| [43] | MAHER Y A, ALI M E A, SALAMA H E, et al. Preparation, characterization and evaluation of chitosan biguanidine hydrochloride as a novel antiscalant during membrane desalination process[J]. Arabian Journal of Chemistry, 2020, 13(1): 2964-2981. DOI: 10.1016/j.arabjc.2018.08.006. |
| [44] | LIU J, WILLFÖR S, XU C L. A review of bioactive plant polysaccharides: biological activities, functionalization, and biomedical applications[J]. Bioactive Carbohydrates and Dietary Fibre, 2015, 5(1): 31-61. DOI: 10.1016/j.bcdf.2014.12.001. |
| [45] |
PRO D, HUGUET S, ARKOUN M, et al. From algal polysaccharides to cyclodextrins to stabilize a urease inhibitor[J]. Carbohydrate Polymers, 2014, 112: 145-151. DOI: 10.1016/j.carbpol.2014.05.075.
pmid: 25129728 |
| [46] | ZHANG H P, LUO X G, LIN X Y, et al. Biodegradable carboxymethyl inulin as a scale inhibitor for calcite crystal growth: Molecular level understanding[J]. Desalination, 2016, 381: 1-7. DOI: 10.1016/j.desal.2015.11.029. |
| [47] | KIRBOGA S, ÖNER M. Investigation of calcium carbonate precipitation in the presence of carboxymethyl inulin[J]. CrystEngComm, 2013, 15(18): 3678-3686. DOI: 10.1039/C3CE27022J. |
| [48] | KIRBOGA S, ÖNER M. The inhibitory effects of carboxymethyl inulin on the seeded growth of calcium carbonate[J]. Colloids and Surfaces B: Biointerfaces, 2012, 91: 18-25. DOI: 10.1016/j.colsurfb.2011.10.031. |
| [49] | BOELS L, WITKAMP G. Carboxymethyl inulin biopolymers: a green alternative for phosphonate calcium carbonate growth inhibitors[J]. Crystal Growth & Design, 2011, 11(9): 4155-4165. DOI: 10.1021/CG2007183. |
| [50] | SHEVCHENKO N M, ANASTYUK S D, GERASIMENKO N I, et al. Polysaccharide and lipid composition of the brown seaweed Laminaria gurjanovae[J]. Russian Journal of Bioorganic Chemistry, 2007, 33(1): 88-98. DOI: 10.1134/S1068162007010116. |
| [51] | OBLUCHINSKAYA E D. Comparative chemical composition of the Barents Sea brown algae[J]. Applied Biochemistry and Microbiology, 2008, 44(3): 305-309. DOI: 10.1134/S0003683808030149. |
| [52] | ITUEN E, AKARANTA O, JAMES A. Evaluation of performance of corrosion inhibitors using adsorption isotherm models: an overview[J]. Chemical Science International Journal, 2017, 18(1): 1-34. DOI: 10.9734/csji/2017/28976. |
| [53] | HE C S, DING R R, CHEN J Q, et al. Interactions between nanoscale zero valent iron and extracellular polymeric substances of anaerobic sludge[J]. Water Research, 2020, 178: 115817. DOI: 10.1016/j.watres.2020.115817. |
| [54] |
YU W, WANG Y W, LI A M, et al. Evaluation of the structural morphology of starch-graft-poly(acrylic acid) on its scale-inhibition efficiency[J]. Water Research, 2018, 141: 86-95. DOI: 10.1016/j.watres.2018.04.021.
pmid: 29778068 |
| [55] | GAO R X, LI Y, ZHU T T, et al. ZIF-8@s-EPS as a novel hydrophilic multifunctional biomaterial for efficient scale inhibition, antibacterial and antifouling in water treatment[J]. The Science of the Total Environment, 2021, 773: 145706. DOI: 10.1016/j.scitotenv.2021.145706. |
| [56] | CHEN G S, HUANG S M, KOU X X, et al. A convenient and versatile amino-acid-boosted biomimetic strategy for the nondestructive encapsulation of biomacromolecules within metal-organic frameworks[J]. Angewandte Chemie International Edition, 2019, 58(5): 1463-1467. DOI: 10.1002/anie.201813060. |
| [57] |
DOONAN C, RICCÒ R, LIANG K, et al. Metal-organic frameworks at the biointerface: synthetic strategies and applications[J]. Accounts of Chemical Research, 2017, 50(6): 1423-1432. DOI: 10.1021/acs.accounts.7b00090.
pmid: 28489346 |
| [58] | OUYANG X P, QIU X Q, LOU H M, et al. Corrosion and scale inhibition properties of sodium lignosulfonate and its potential application in recirculating cooling water system[J]. Industrial & Engineering Chemistry Research, 2006, 45(16): 5716-5721. DOI: 10.1021/ie0513189. |
| [59] | 张惠欣, 葛丽环, 周宏勇, 等. 羧烷基-季铵两性壳聚糖的制备及其阻垢杀菌性能[J]. 化工进展, 2011, 30(9): 2055-2059. DOI: 10.16085/j.issn.1000-6613.2011.09.016. |
| [60] | GUO X R, QIU F X, DONG K, et al. Scale inhibitor copolymer modified with oxidized starch: synthesis and performance on scale inhibition[J]. Polymer-Plastics Technology and Engineering, 2013, 52(3): 261-267. DOI: 10.1080/03602559.2012.747206. |
| [61] | GONCHARUK V V, KAVITSKAYA A A, SKILSKAYA M D. Sodium carboxymethyl cellulose as an inhibitor of scale formation in nanofiltration of hard artesian waters[J]. Desalination and Water Treatment, 2012, 47(1/2/3): 235-242. DOI: 10.1080/19443994.2012.696408. |
| [62] | YU W, SONG D, LI A, et al. Control of gypsum-dominated scaling in reverse osmosis system using carboxymethyl cellulose[J]. Journal of Membrane Science, 2019, 577: 20-30. DOI: 10.1016/j.memsci.2019.01.053. |
| [63] | WANG Y, LI A, YANG H. Effects of substitution degree and molecular weight of carboxymethyl starch on its scale inhibition[J]. Desalination, 2017, 408: 60-69. DOI: 10.1016/j.desal.2017.01.006. |
| [64] | PRISCIANDARO M, MAZZIOTTI DI CELSO G, LANCIA A, et al. Citric acid as a green additive to retard calcium carbonate scales on process equipment[J]. The Canadian Journal of Chemical Engineering, 2020, 98(9): 1973-1979. DOI: 10.1002/cjce.23783. |
| [65] | SINN C G, DIMOVA R, ANTONIETTI M. Isothermal titration calorimetry of the polyelectrolyte/water interaction and binding of Ca2+: effects determining the quality of polymeric scale inhibitors[J]. Macromolecules, 2004, 37(9): 3444-3450. DOI: 10.1021/ma030550s. |
| [66] | WADA N, YAMASHITA K, UMEGAKI T. Effects of carboxylic acids on calcite formation in the presence of Mg2+ ions[J]. Journal of Colloid and Interface Science, 1999, 212(2): 357-364. DOI: 10.1006/jcis.1998.6067. |
| [67] | GHIZELLAOUI S, SEMINERAS H. Inhibition of scale formation by electrochemical means in the presence of a green inhibitor: citric acid[J]. Journal of Materials and Environmental Science, 2017, 8(6): 2105-2111. |
| [68] | YUAN X J, DONG S Y, ZHENG Q, et al. Novel and efficient curcumin based fluorescent polymer for scale and corrosion inhibition[J]. Chemical Engineering Journal, 2020, 389: 124296. DOI: 10.1016/j.cej.2020.124296. |
| [69] | AL-SABAGH A M, EL BASIONY N M, SADEEK S A, et al. Scale and corrosion inhibition performance of the newly synthesized anionic surfactant in desalination plants: experimental, and theoretical investigations[J]. Desalination, 2018, 437: 45-58. DOI: 10.1016/j.desal.2018.01.036. |
| [70] | ZHANG W W, LI H J, CHEN L W, et al. Performance and mechanism of a composite scaling-corrosion inhibitor used in seawater: 10-Methylacridinium iodide and sodium citrate[J]. Desalination, 2020, 486: 114482. DOI: 10.1016/j.desal.2020.114482. |
| [71] | TSUTSUMI N, OYA M, SAKAI W. Biodegradable network polyesters from gluconolactone and citric acid[J]. Macromolecules, 2004, 37(16): 5971-5976. DOI: 10.1021/ma049607g. |
| [72] |
DU Q, WANG Y, LI A, et al. Scale-inhibition and flocculation dual-functionality of poly(acrylic acid) grafted starch[J]. Journal of Environmental Management, 2018, 210: 273-279. DOI: 10.1016/j.jenvman.2018.01.016.
pmid: 29353116 |
| [73] |
YU Y, WANG Y N, DING W, et al. Preparation of highly-oxidized starch using hydrogen peroxide and its application as a novel ligand for zirconium tanning of leather[J]. Carbohydrate Polymers, 2017, 174: 823-829. DOI: 10.1016/j.carbpol.2017.06.114.
pmid: 28821137 |
| [74] | CHEN X S, CHEN Y, CUI J J, et al. Molecular dynamics simulation and DFT calculation of “green” scale and corrosion inhibitor[J]. Computational Materials Science, 2021, 188: 110229. DOI: 10.1016/j.commatsci.2020.110229. |
| [75] | BUTLER M F, GLASER N, WEAVER A C, et al. Calcium carbonate crystallization in the presence of biopolymers[J]. Crystal Growth & Design, 2006, 6(3): 781-794. DOI: 10.1021/cg050436w. |
| [76] | DIETZSCH M, BARZ M, SCHÜLER T, et al. PAA-PAMPS copolymers as an efficient tool to control CaCO3 scale formation[J]. Langmuir: the ACS Journal of Surfaces and Colloids, 2013, 29(9): 3080-3088. DOI: 10.1021/la4000044. |
| [77] | KAZI S N, DUFFY G G, CHEN X D. Fouling mitigation of heat exchangers with natural fibres[J]. Applied Thermal Engineering, 2013, 50(1): 1142-1148. DOI: 10.1016/j.applthermaleng.2012.08.042. |
| [78] | WANG C, ZHU D Y, WANG X K. Low-phosphorus maleic acid and sodium ρ-styrenesulfonate copolymer as calcium carbonate scale inhibitor[J]. Journal of Applied Polymer Science, 2010, 115(4): 2149-2155. DOI: 10.1002/app.31300. |
| [79] | LOPEZ C G, ROGERS S E, COLBY R H, et al. Structure of sodium carboxymethyl cellulose aqueous solutions: A SANS and rheology study[J]. Journal of Polymer Science Part B, Polymer Physics, 2015, 53(7): 492-501. DOI: 10.1002/polb.23657. |
| [80] | JEDVERT K, HEINZE T. Cellulose modification and shaping: a review[J]. Journal of Polymer Engineering, 2017, 37(9): 845-860. DOI: 10.1515/polyeng-2016-0272. |
| [81] | LI W Z, HUANG S Y, XU D J, et al. Molecular dynamics simulations of the characteristics of sodium carboxymethyl cellulose with different degrees of substitution in a salt solution[J]. Cellulose, 2017, 24(9): 3619-3633. DOI: 10.1007/s10570-017-1364-0. |
| [82] | SHUI T, FENG S H, CHEN G, et al. Synthesis of sodium carboxymethyl cellulose using bleached crude cellulose fractionated from cornstalk[J]. Biomass and Bioenergy, 2017, 105: 51-58. DOI: 10.1016/j.biombioe.2017.06.016. |
| [83] | TENG K H, KAZI S N, AMIRI A, et al. Calcium carbonate fouling on double-pipe heat exchanger with different heat exchanging surfaces[J]. Powder Technology, 2017, 315: 216-226. DOI: 10.1016/j.powtec.2017.03.057. |
| [84] | XU Z M, ZHAO Y, HE J J, et al. Fouling characterization of calcium carbonate on heat transfer surfaces with sodium carboxymethyl cellulose as an inhibitor[J]. International Journal of Thermal Sciences, 2021, 162: 106790. DOI: 10.1016/j.ijthermalsci.2020.106790. |
| [85] | YU W, CHEN W, YANG H. Evaluation of structural effects on the antiscaling performance of various graft cellulose-based antiscalants in RO membrane scaling control[J]. Journal of Membrane Science, 2021, 620: 118893. DOI: 10.1016/j.memsci.2020.118893. |
| [86] | QIANG X, SHENG Z, ZHANG H. Study on scale inhibition performances and interaction mechanism of modified collagen[J]. Desalination, 2013, 309: 237-242. DOI: 10.1016/j.desal.2012.10.025. |
| [87] | HU P, XI Z, LI Y, et al. Evaluation of the structural factors for the flocculation performance of a co-graft cationic starch-based flocculant[J]. Chemosphere, 2020, 240: 124866. DOI: 10.1016/j.chemosphere.2019.124866. |
| [88] | MISHRA S, SAXENA P, DEORE D A. Studies on antiscaling effect of polyacrylic acid on boiler[J]. Polymer-Plastics Technology and Engineering, 2005, 44(8/9): 1389-1398. DOI: 10.1080/03602550500209754. |
| [89] | YANG Q F, GU A Z, DING J, et al. Effects of PAA and PBTCA on CaCO3 scaling in pool boiling system[J]. Chinese Journal of Chemical Engineering, 2002, 10(2): 190-197. |
| [90] | ZHAO Y, XU Z M, WANG B B, et al. Scale inhibition performance of sodium carboxymethyl cellulose on heat transfer surface at various temperatures: Experiments and molecular dynamics simulation[J]. International Journal of Heat and Mass Transfer, 2019, 141: 457-463. DOI: 10.1016/j.ijheatmasstransfer.2019.06.091. |
| [91] |
BOLTO B, GREGORY J. Organic polyelectrolytes in water treatment[J]. Water Research, 2007, 41(11): 2301-2324. DOI: 10.1016/j.watres.2007.03.012.
pmid: 17462699 |
| [92] | RINAUDO M. Chitin and chitosan: Properties and applications[J]. ChemInform, 2007, 38(27): 603-632. DOI: 10.1002/chin.200727270. |
| [93] |
ZHU F. Composition, structure, physicochemical properties, and modifications of cassava starch[J]. Carbohydrate Polymers, 2015, 122: 456-480. DOI: 10.1016/j.carbpol.2014.10.063.
pmid: 25817690 |
| [94] |
YANG R, LI H J, HUANG M, et al. A review on chitosan-based flocculants and their applications in water treatment[J]. Water Research, 2016, 95: 59-89. DOI: 10.1016/j.watres.2016.02.068.
pmid: 26986497 |
| [95] | SHAK K P Y, WU T Y. Synthesis and characterization of a plant-based seed gum via etherification for effective treatment of high-strength agro-industrial wastewater[J]. Chemical Engineering Journal, 2017, 307: 928-938. DOI: 10.1016/j.cej.2016.08.045. |
| [96] | ZHANG H X, CAI Z Y, JIN X H, et al. Preparation of modified oligochitosan and evaluation of its scale inhibition and fluorescence properties[J]. Journal of Applied Polymer Science, 2015, 132(37): 42518. DOI: 10.1002/app.42518. |
| [97] | ZENG D F, CHEN T S, ZHOU S J. Synthesis of polyaspartic acid/chitosan graft copolymer and evaluation of its scale inhibition and corrosion inhibition performance[J]. International Journal of Electrochemical Science, 2015, 10(11): 9513-9527. DOI: 10.1016/S1452-3981(23)11197-7. |
| [98] | ZOU Z Y, BERTINETTI L, POLITI Y, et al. Control of polymorph selection in amorphous calcium carbonate crystallization by poly(aspartic acid): two different mechanisms[J]. Small (Weinheim an Der Bergstrasse, Germany), 2017, 13(21): 10.1002/smll.201603100. DOI: 10.1002/smll.201603100. |
| [99] | PRAMANIK B K, GAO Y H, FAN L H, et al. Antiscaling effect of polyaspartic acid and its derivative for RO membranes used for saline wastewater and brackish water desalination[J]. Desalination, 2017, 404: 224-229. DOI: 10.1016/j.desal.2016.11.019. |
| [100] | HASSON D, SHEMER H, SHER A. State of the art of friendly “green” scale control inhibitors: a review article[J]. Industrial & Engineering Chemistry Research, 2011, 50(12): 7601-7607. DOI: 10.1021/ie200370v. |
| [101] | CHHIM N, HADDAD E, NEVEUX T, et al. Performance of green antiscalants and their mixtures in controlled calcium carbonate precipitation conditions reproducing industrial cooling circuits[J]. Water Research, 2020, 186: 116334. DOI: 10.1016/j.watres.2020.116334. |
| [102] | MITHIL KUMAR N, GUPTA S K, JAGADEESH D, et al. Development of poly(aspartic acid-co-malic acid) composites for calcium carbonate and sulphate scale inhibition[J]. Environmental Technology, 2015, 36(10): 1281-1290. DOI: 10.1080/09593330.2014.984773. |
| [103] | MARTINOD A, NEVILLE A, EUVRAD M, et al, et al. Electrodeposition of a calcareous layer: effects of green inhibitors[J]. Chemical Engineering Science, 2009, 64(10):2413-2421.DOI:10.1016/j.ces.2009.01.024. |
| [104] | QUAN Z H, CHEN Y C, WANG X R, et al. Experimental study on scale inhibition performance of a green scale inhibitor polyaspartic acid[J]. Science in China Series B: Chemistry, 2008, 51(7): 695-699. DOI: 10.1007/s11426-008-0063-y. |
| [105] | 吴新世, 孙波, 王菁, 等. 一种新型高聚物生物可降解性评价[J]. 南开大学学报(自然科学版), 2009, 42(4): 13-17. |
| [106] | TOUIR R, CENOUI M, BAKRI M E, et al. Sodium gluconate as corrosion and scale inhibitor of ordinary steel in simulated cooling water[J]. Corrosion Science, 2008, 50(6): 1530-1537. DOI: 10.1016/j.corsci.2008.02.011. |
| [107] | LING L, ZHOU Y M, HUANG J Y, et al. Carboxylate-terminated double-hydrophilic block copolymer as an effective and environmental inhibitor in cooling water systems[J]. Desalination, 2012, 304: 33-40. DOI: 10.1016/j.desal.2012.07.014. |
| [108] | CHEN J X, XU L H, HAN J, et al. Synthesis of modified polyaspartic acid and evaluation of its scale inhibition and dispersion capacity[J]. Desalination, 2015, 358: 42-48. DOI: 10.1016/j.desal.2014.11.010. |
| [109] | ZHANG Y, YIN H Q, ZHANG Q S, et al. Synthesis and characterization of novel polyaspartic acid/urea graft copolymer with acylamino group and its scale inhibition performance[J]. Desalination, 2016, 395: 92-98. DOI: 10.1016/j.desal.2016.05.020. |
| [110] | 余吉良, 王志坤, 霍然, 等. 弱碱环境中碳酸钙垢阻垢剂的阻垢性能与阻垢机理[J]. 油田化学, 2017, 34(4): 699-704. DOI: 10.19346/j.cnki.1000-4092.2017.04.026. |
| [111] | ZHAO L N, ZHOU Y M, YAO Q Z, et al. Calcium scale inhibition of stimulated oilfield produced water using polyaspartic acid/aminomethanesulfonic acid[J]. ChemistrySelect, 2021, 6(15): 3692-3701. DOI: 10.1002/slct.202100853. |
| [112] | CHEN J X, CHEN F J, HAN J, et al. Evaluation of scale and corrosion inhibition of modified polyaspartic acid[J]. Chemical Engineering & Technology, 2020, 43(6): 1048-1058. DOI: 10.1002/ceat.201900518. |
| [113] |
GUO X Y, ZHAO X W, XU Y H, et al. The synthesis of polyaspartic acid derivative PASP-Im and investigation of its scale inhibition performance and mechanism in industrial circulating water[J]. RSC Advances, 2020, 10(55): 33595-33601. DOI: 10.1039/d0ra06592g.
pmid: 35515019 |
| [114] | ZHANG S P, QU H J, YANG Z, et al. Scale inhibition performance and mechanism of sulfamic/amino acids modified polyaspartic acid against calcium sulfate[J]. Desalination, 2017, 419: 152-159. DOI: 10.1016/j.desal.2017.06.016. |
| [115] | ZHANG B R, HE C J, WANG C, et al. Synergistic corrosion inhibition of environment-friendly inhibitors on the corrosion of carbon steel in soft water[J]. Corrosion Science, 2015, 94: 6-20. DOI: 10.1016/j.corsci.2014.11.035. |
| [116] | XU Y, ZHAO L L, WANG L N, et al. Synthesis of polyaspartic acid-melamine grafted copolymer and evaluation of its scale inhibition performance and dispersion capacity for ferric oxide[J]. Desalination, 2012, 286: 285-289. DOI: 10.1016/j.desal.2011.11.036. |
| [117] | CHEN Y, CHEN X S, LIANG Y N. Synthesis of polyaspartic acid/graphene oxide grafted copolymer and evaluation of scale inhibition and dispersion performance[J]. Diamond and Related Materials, 2020, 108: 107949. DOI: 10.1016/j.diamond.2020.107949. |
| [118] | LI C, ZHANG C, ZHANG W. The inhibition effect mechanisms of four scale inhibitors on the formation and crystal growth of CaCO3 in solution[J]. Scientific Reports, 2019, 9(1): 13366.DOI:10.1038/s41598-019-50012-7. |
| [119] | ZHOU X H, SUN Y H, WANG Y Z. Inhibition and dispersion of polyepoxysuccinate as a scale inhibitor[J]. Journal of Environmental Sciences, 2011, 23: S159-S161. DOI: 10.1016/S1001-0742(11)61102-9. |
| [120] | LIU C, ZHENG Y F, YANG S Y, et al. Exploration of a novel depressant polyepoxysuccinic acid for the flotation separation of pentlandite from lizardite slimes[J]. Applied Clay Science, 2021, 202: 105939. DOI: 10.1016/j.clay.2020.105939. |
| [121] | SHI W Y, XU W, CANG H, et al. Design and synthesis of biodegradable antiscalant based on MD simulation of antiscale mechanism: a case of itaconic acid-epoxysuccinate copolymer[J]. Computational Materials Science, 2017, 136: 118-125. DOI: 10.1016/j.commatsci.2017.04.035. |
| [122] | ZUO Y W, YANG W Z, ZHANG K G, et al. Experimental and theoretical studies of carboxylic polymers with low molecular weight as inhibitors for calcium carbonate scale[J]. Crystals, 2020, 10(5): 406. DOI: 10.3390/cryst10050406. |
| [123] | LI C J, ZHANG C Y, ZHANG W P. The inhibitory effects of four inhibitors on the solution adsorption of CaCO3 on Fe3O4 and Fe2O3 surfaces[J]. Scientific Reports, 2019, 9: 13724. DOI: 10.1038/s41598-019-50127-x. |
| [124] | HUANG H H, YAO Q, JIAO Q, et al. Polyepoxysuccinic acid with hyper-branched structure as an environmentally friendly scale inhibitor and its scale inhibition mechanism[J]. Journal of Saudi Chemical Society, 2019, 23(1): 61-74. DOI: 10.1016/j.jscs.2018.04.003. |
| [125] | ZHANG K F, CHEN F J, HAN J, et al. Evaluation of arginine-modified polyepoxysuccinic acid as anti-scaling and anti-corrosion agent[J]. Chemical Engineering & Technology, 2021, 44(6): 1131-1140. DOI: 10.1002/ceat.202000576. |
| [126] | HUANG H H, YAO Q, LIU B L, et al. Synthesis and characterization of scale and corrosion inhibitors with hyper-branched structure and the mechanism[J]. New Journal of Chemistry, 2017, 41(20): 12205-12217. DOI: 10.1039/C7NJ02201H. |
| [127] |
YAN M F, TAN Q Q, LIU Z, et al. Synthesis and application of a phosphorous-free and non-nitrogen polymer as an environmentally friendly scale inhibition and dispersion agent in simulated cooling water systems[J]. ACS Omega, 2020, 5(25): 15487-15494. DOI: 10.1021/acsomega.0c01620.
pmid: 32637823 |
| [128] | WAN C, WANG L T, SHA J Y, et al. Effect of carbon nanoparticles on the crystallization of calcium carbonate in aqueous solution[J]. Nanomaterials (Basel, Switzerland), 2019, 9(2): 179. DOI: 10.3390/nano9020179. |
| [129] | TENG K H, AMIRI A, KAZI S N, et al. Fouling mitigation on heat exchanger surfaces by EDTA-treated MWCNT-based water nanofluids[J]. Journal of the Taiwan Institute of Chemical Engineers, 2016, 60: 445-452. DOI: 10.1016/j.jtice.2015.11.006. |
| [130] | HAO J, LI L Y, ZHAO W W, et al. Synthesis and application of CCQDs as a novel type of environmentally friendly scale inhibitor[J]. ACS Applied Materials & Interfaces, 2019, 11(9): 9277-9282. DOI: 10.1021/acsami.8b19015. |
| [131] | ALROOMI Y M, HUSSAIN K F. Potential kinetic model for scaling and scale inhibition mechanism[J]. Desalination, 2016, 393: 186-195. DOI: 10.1016/j.desal.2015.07.025. |
| [132] | AMJAD Z. Mineral scales in biological and industrial systems[J]. Crc Press, 2013, 10.1201/b1: 77-102. |
| [133] | RAHMAN F. Calcium sulfate precipitation studies with scale inhibitors for reverse osmosis desalination[J]. Desalination, 2013, 319: 79-84. DOI: 10.1016/j.desal.2013.03.027. |
| [134] |
DOBBERSCHÜTZ S, NIELSEN M R, SAND K K, et al. The mechanisms of crystal growth inhibition by organic and inorganic inhibitors[J]. Nature Communications, 2018, 9: 1578. DOI: 10.1038/s41467-018-04022-0.
pmid: 29679006 |
| [135] | ZHENG Z, YU Z P, YANG M D, et al. Substituent group variations directing the molecular packing, electronic structure, and aggregation-induced emission property of isophorone derivatives[J]. The Journal of Organic Chemistry, 2013, 78(7): 3222-3234. DOI: 10.1021/jo400116j. |
| [1] | LIU Ting,ZHAO Chang-sheng,CHEN Qing-feng,SI Guo-rui,LI Lei,FENG You,LI Jin-ye. Coagulation pretreatment of concentrated liquid behind landfill leachate membrane [J]. Shandong Science, 2022, 35(1): 115-119. |
| [2] | PENG Li-Min, YAN Zhong-Yue, SHAO Bo, ZHAO Jian-Jian. Analysis of water pollution caused by the development of three industries in Nansi Lake basin [J]. J4, 2012, 25(2): 33-37. |
|
||

Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License (CC BY-NC 4.0), which permits third parties to freely share (i.e., copy and redistribute the material in any medium or format) and adapt (i.e., remix, transform, or build upon the material) the articles published in this journal, provided that appropriate credit is given, a link to the license is provided, and any changes made are indicated. The material may not be used for commercial purposes. For details of the CC BY-NC 4.0 license, please visit: https://creativecommons.org/licenses/by-nc/4.0